The Evolution of Modern Cardiovascular Medicine
The landscape of cardiovascular drug development has undergone remarkable transformation in recent decades, reflecting both scientific advances and changing patient needs. From traditional therapies to groundbreaking molecular innovations, the field continues to evolve at an unprecedented pace. This shift represents not just scientific progress, but a fundamental change in how we approach heart health and disease prevention.
Today's cardiovascular drug development landscape integrates cutting-edge technologies, artificial intelligence, and deep understanding of genetic factors. Researchers and pharmaceutical companies are investing billions in developing more effective, targeted treatments that promise better outcomes with fewer side effects. This renaissance in cardiovascular medicine is reshaping treatment paradigms and offering new hope to millions of patients worldwide.
Breakthrough Technologies Reshaping Treatment Approaches
Artificial Intelligence and Machine Learning Integration
The integration of artificial intelligence in cardiovascular drug development has revolutionized how researchers identify and validate potential drug candidates. Machine learning algorithms can now analyze vast datasets of molecular structures, patient responses, and clinical trials, significantly accelerating the drug discovery process. This technology enables scientists to predict drug interactions and efficacy with unprecedented accuracy, reducing development timelines and costs.
Advanced AI systems are also helping to identify novel drug targets by analyzing complex biological networks and pathways involved in cardiovascular disease. This sophisticated approach has led to the discovery of several promising compounds that might have been overlooked using traditional research methods.
Precision Medicine and Genetic Targeting
The emergence of precision medicine has fundamentally altered cardiovascular drug development strategies. By understanding individual genetic profiles, researchers can now develop treatments tailored to specific patient populations. This approach has led to more effective therapies with reduced side effects, as treatments are matched to patients most likely to respond positively.
Genetic targeting has enabled the development of drugs that address previously untreatable cardiovascular conditions. Scientists can now identify specific genetic mutations associated with heart disease and design molecules that precisely target these abnormalities.
Novel Therapeutic Approaches and Mechanisms
RNA-Based Therapeutics
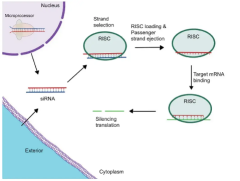
RNA-based therapeutics represent one of the most promising frontiers in cardiovascular drug development. These innovative treatments can modify gene expression, potentially treating conditions at their source rather than just managing symptoms. The success of RNA technologies in other therapeutic areas has sparked intense interest in their application to cardiovascular disease.
Several pharmaceutical companies are currently developing RNA-based treatments for conditions like high cholesterol, heart failure, and rare genetic cardiovascular disorders. These therapies offer the potential for longer-lasting effects and may require less frequent dosing compared to traditional medications.
Cell and Gene Therapy Innovations
The field of cell and gene therapy has opened new possibilities in cardiovascular drug development. Scientists are exploring ways to repair damaged heart tissue, regenerate blood vessels, and correct genetic defects that lead to heart disease. These approaches could potentially offer curative treatments rather than just symptom management.
Recent advances in delivery systems and genetic modification techniques have made these therapies more practical and safer for clinical use. The success of early trials has encouraged increased investment in this promising area of research.
Patient-Centric Development Strategies
Real-World Evidence Integration
Modern cardiovascular drug development increasingly relies on real-world evidence to complement traditional clinical trials. This approach provides valuable insights into how treatments perform in diverse patient populations and real-life settings. Researchers are using data from electronic health records, wearable devices, and patient registries to inform drug development decisions.
This shift towards real-world evidence has led to more efficient trial designs and better understanding of treatment effectiveness across different patient subgroups. It has also helped identify previously unknown side effects and interactions that might not be apparent in controlled clinical trials.
Digital Health Technology Integration
Digital health technologies are revolutionizing how cardiovascular drugs are developed and tested. Wearable devices and mobile applications provide continuous monitoring of patient vital signs and symptoms, offering unprecedented insight into treatment efficacy. This real-time data collection enables more accurate assessment of drug effects and patient responses.
The integration of digital health tools has also improved patient engagement in clinical trials and made it easier to track long-term outcomes. These technologies are helping researchers design more efficient studies and gather more comprehensive data about drug performance.
Regulatory and Market Access Considerations
Accelerated Approval Pathways
Regulatory bodies have developed new pathways to expedite the approval of promising cardiovascular treatments. These accelerated programs help bring innovative therapies to patients more quickly while maintaining rigorous safety standards. Companies engaged in cardiovascular drug development are increasingly utilizing these pathways to speed up the development process.
The success of these programs has encouraged more investment in cardiovascular research and development, particularly for treatments addressing unmet medical needs. This has led to a more dynamic and responsive drug development ecosystem.
Value-Based Pricing Models
The shift towards value-based healthcare has influenced how cardiovascular drugs are developed and commercialized. Developers must now demonstrate not just clinical efficacy but also economic value to healthcare systems. This has led to increased focus on treatments that can reduce hospitalizations and improve long-term outcomes.
Companies are incorporating health economics and outcomes research earlier in the development process to ensure their products meet both clinical and economic objectives. This approach helps secure market access and reimbursement for new treatments.
Frequently Asked Questions
What are the main challenges in cardiovascular drug development today?
The primary challenges include the length and cost of clinical trials, the complexity of cardiovascular disease mechanisms, and the need to demonstrate significant improvements over existing treatments. Additionally, researchers must address safety concerns and potential long-term effects while working within strict regulatory frameworks.
How long does it typically take to develop a new cardiovascular drug?
The development of a new cardiovascular drug typically takes 10-15 years from initial discovery to market approval. This includes preclinical research, multiple phases of clinical trials, regulatory review, and post-market surveillance. The process requires significant investment and involves rigorous safety and efficacy testing.
What role do biomarkers play in cardiovascular drug development?
Biomarkers are crucial in cardiovascular drug development as they help identify potential drug targets, monitor treatment effectiveness, and predict patient responses. They enable more precise patient selection for clinical trials and support the development of personalized treatment approaches. Advanced biomarker research has significantly improved the efficiency of drug development processes.

